Kiran Sury interviews scientists exploring the emerging field of mechanobiology, which could lead to cures for a broad range of diseases. (Kiran Sury)
By KIRAN SURY
There is never a good time to learn that you have diabetes. First come the metformin pills, soon replaced by nightly insulin injections. When all else fails, you face the ignominy of hours on a dialysis machine three times a week.
But in the future, you might not have to go through any of that.
The doctor would administer the treatment immediately: one injection of ferromagnetic fluid, right into the bloodstream. The tiny iron particles latch on to your fat and muscle cells, where they sway back and forth under the force of a tightly controlled magnetic field generated by an electromagnet. The calibrated pulling and prodding from this force directs your cells to make more surface receptors, increasing your sensitivity to insulin and lowering your blood sugar to normal levels. There is never a good time to learn that you have diabetes, but in the year 2050 the news is not so bad.
This medical scenario could be straight out of science fiction. It imagines a kind of internal massage that slowly cures disease. Thanks to the oft-neglected field of mechanobiology, an emerging scientific realm that focuses on the way physical forces can change cells, such a force-based treatment is increasingly becoming a possibility.
Researchers are learning the importance of the forces cells exert on each other and their environment. Studying these forces could lead to new research that helps regrow organs, fight cancer and prevent bacteria from spreading disease. The science is still in its infancy, and it may take more than 30 years, but curing disease could one day be as simple as tugging at our cells in the right place at the right time.
“The problem is a very complex one,” said Dr. Michael Sheetz, a decorated mechanobiologist who studies cell dynamics at Columbia University and founded both the Nanomedicine Center for Mechanobiology and the Mechanobiology Institute in Singapore. But, he later added, “there may be a situation where a mechanical treatment could provide benefit. I would hope so.”
While chemistry has long been intertwined with the biological branch of science, physics is a relatively more recent addition. Almost a century ago, mathematical biologist D’Arcy Thompson noted the importance of forces to biological development. In his seminal “On Growth and Form,” he writes, “Cell and tissue, shell and bone, leaf and flower, are so many portions of matter, and it is in obedience to the laws of physics that their particles have been moved, moulded and conformed.”
In the 1950s, Andrew and Hugh Huxley, two unrelated scientists who happened to be working on the same subject at the same time, discovered how the microscopic protein filaments actin and myosin slide past each other to produce muscle contraction. This chemically powered process generates muscular force, which in turn activates other signaling pathways. This is mechanotransduction: the conversion of mechanical forces to chemical signals to produce change in cells. For instance, in the ear, sound waves apply pressure to hair cells. That’s a mechanical force. The hair cells convert that force into chemical signals, opening ion channels and sending a signal to the brain via the nervous system. The cells sense the mechanical change, and generate further chemical signals as a response.
The technology needed to sense forces at a sub-cellular level have slowly been refined over the past two decades, leading to more interdisciplinary research and a realization that the mechanical aspects of biology are as important as the chemical. Scientists use “tweezers” made of laser beams or magnetic fields to tug on cells and feel them pull back. Atomic-force microscopy can probe the surface of cell structures measured in nanometers.
With the ability to work on an unprecedentedly small scale, scientists have found out that cells react differently when placed on a surface made of submicron pillars. Researchers borrow the same techniques used to print computer chips to manufacture an array of columns, each a hundred times thinner than the width of a human hair. The novelty of the surface forces the cells to adhere in a new way, clinging to the top of the pillars rather than spreading around the sides. It’s akin to the difference between standing in the middle of a sidewalk rather than balancing on the curb – when forced to the edge, cells plant themselves on the top of the pillars for greater stability.
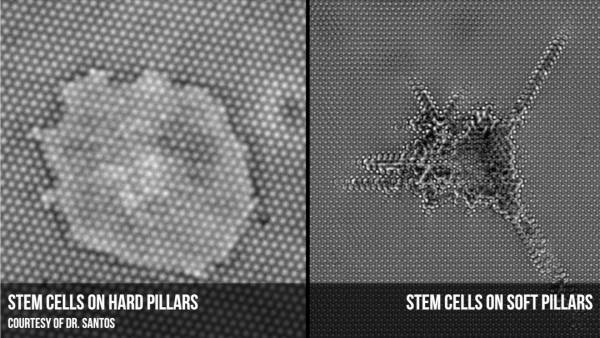
Working in an environment measured in billionths of a meter has helped reveal details like this that would be lost on a larger scale, said Dr. Saba Ghassemi, a mechanical engineer who made the specialized surfaces used in the research. “We can mimic exactly what the native environment of a cell is,” Dr. Ghassemi, who divides her time between Columbia University and the University of Pennsylvania, said in a telephone interview. “It gives medicine or biology an opportunity to understand precisely what is happening.”
In the past, scientists attended closely to the type of solution in which stem cells were grown. Getting cells to differentiate-–to grow into a heart rather than a kidney, for example – is a complicated process. Specific chemical brews of signaling molecules and growth factors are used to spur cells into their final forms, but it can be tricky to get the mix just right. This is of particular importance to stem cells, which have the potential to treat damaged organs or grow replacements from scratch.
Now researchers are also focusing on the rigidity of the substrate—the fancy name for the surfaces on which cells are grown, often inflexible plastic or glass plates. Mechanobiologists have found that using softer surfaces can have a large effect on cell behavior.
“To grow cells in a dish is highly inefficient,” said Luis Santos, who recently received his doctorate in biology at Columbia University. “There is nothing in your body that resembles a plastic dish.”
Scientists at the University of Pennsylvania put stem cells in the same nutrient broth but on polymers with easily tweaked rigidities to see how it affected cell differentiation. Cells placed on a mushy substrate that felt a lot like brain matter spread out and made brain-like connections. A medium, muscle-like surface gave rise to long, spindly muscle cells, while a hard bottom made bone cells. To put it in human terms, it’s the equivalent of switching from a hard mattress to a softer one and waking up with a different appearance. The rigidity of adjacent surfaces, it turns out, matters a lot to fledgling cells.
“Those forces are critical in order to get the right cascade of genes being expressed,” said Brooklyn College mechanobiologist Dr. Nicolas Biais.
This rigidity preference could explain some of the difficulty researchers are having with actual treatments. Stem cells injected after a heart attack, the theory goes, should grow into muscle cells and restore function. But the rigid scar tissue present after a cardiac arrest could interfere and prevent the cells from differentiating correctly.
“If you don’t pay attention to this you could just accidentally set up a system that’s impossible because you’re providing all the right chemical signals but the mechanical environment is wrong,” said Dr. Michael Dustin, director of the Nanomedicine Center for Mechanobiology, an international collaboration that includes New York and Columbia University.
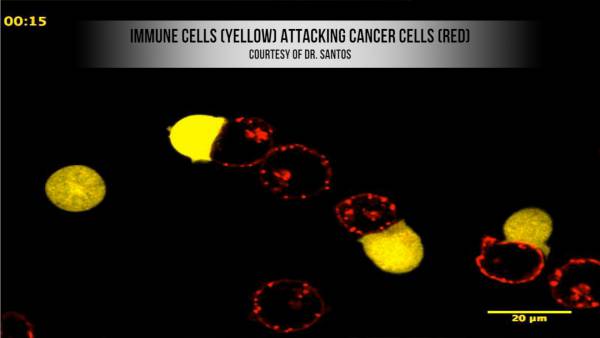
Dr. Dustin, an immunologist by training, has also been working with physicians at the University of Pennsylvania on using the effects of rigidity to improve cancer treatments. Drs. Carl June and Michael Milone use adoptive cell therapy to cure leukemia. The technique involves removing t-cells, white blood cells that are part of the immune system, from the patient and growing them up outside the body. The cells are then genetically modified to target the cancer and injected back into the patient.
The adaptability of adoptive cell therapy makes it a very promising treatment. T-cells by their nature are very specific, and studies are underway to see if different protein markers can be used to target different types of cancer. That means fewer deleterious side effects than traditional chemotherapy, which targets all rapidly dividing cells and attacks the bone marrow and hair as well as cancer cells.
But the process is bottlenecked by the fact that leukemia patients have a very low t-cell count. T-cells replicate in the body, so a single injection should be enough to eliminate a tumor. But patients need a high enough initial amount to kick start the process. Finding a way to make more of them could make a big difference for patients with practically no immune system.
This is where the insights gained from mechanobiology can come into play. By combining the necessary growth factors with a pliable silicone substrate rather than rigid plastic, Dr. Dustin has found that it is possible to quadruple the number of cells grown in the same amount of time. The t-cells react favorably to the suppleness of the silicone, which is more like their natural environment. “We basically wanted to combine mechanical cues with those chemical signals to try and optimize that process,” said Dr. Dustin.
The next step is to see if the increased number of cells actually makes a difference in the body. “This might have benefit to immunotherapy if the number of cells are limiting,” Dr. Milone wrote in an email interview. “The cells persist which may provide long term tumor control if eradication of the tumor is not possible.”
The exact mechanisms by which cells sense the stiffness of their environment and convert that into cellular change are unknown, but scientists think it has a lot to do with tensegrity. Short for tensional integrity, tensegrity is the architectural idea that tension and compression can complement each other to provide structural stability and flexibility. Unlike other rigid structures like the keystone of an arch that rely on tension, tensegrity structures can stretch. First applied to biology by Harvard bioengineer Donald Ingber in 1998, it fits neatly with the cytoskeleton, a network of filaments within the cell that acts as both its backbone and transport system.
Dr. Dennis Discher, who led the University of Pennsylvania stem cell team and called mechanobiology “essential” to his research, has also had some success in elucidating exactly how mechanical signals from the outside of a cell propagate through the cytoskeleton to cause cellular changes.
When a cell lands on a new environment, it adheres via integrins, receptors that span the cellular membrane. Integrins serve a dual purpose as both anchor and antennae, as they attach to the cell’s environment and help transmit the resulting external signal through the cytoskeleton all the way to the nucleus. Discher has found that for stem cells, the increased stiffness from a harder surface leads to increasing stiffness within the cell’s nucleus; this physically alters access to DNA and redirects the construction of cellular components. This could lead the cells to absorb more minerals as they turn into bone, matching the stiffness of their surface.
Cell receptors like integrins are important because their function is impaired for cancerous cells. “Cancer is really a mechanically driven disease that is the inappropriate response of the cell to its environment,” Dr. Sheetz explained. Cancer cells don’t follow the rules when it comes to cellular movement and adhesion, which enables tumors to metastasize and spread to different organs.
Integrins and other molecules associated with cell attachment and movement could be used to target and inhibit cancer cells. Mina Bissell, a pioneer in research on cells and their microenvironment, found that breast cancer cells treated with integrin inhibitors interacted differently with their surroundings and reverted to normal cells. “This is the idea that you can actually compensate for a defect in your [DNA] by your environment, your mechanical environment,” Dr. Biais said.
Dr. Biais heads up the Mechano-Microbiology lab at Brooklyn College, where he studies how the pathogen that causes gonorrhea interacts with human cells. Neisseria gonorrhea has specialized tendril-like appendages called type-four pili that it uses to tug on cell membranes. The pili attach and retract with such strength and speed that Dr. Biais has dubbed the organism a “micro-scale Spider-man.”
For the human body, however, N. gonorrhea is more super-villain than superhero. It causes the well-known sexually transmitted disease, and the Centers for Disease Control has labeled certain antibiotic-resistant strains as an urgent threat to public health. Dr. Biais collaborates with a University of Arizona lab that has found that disabling the molecular motor governing the pili retraction makes the bacteria noninfectious.
“It could be that Neisseria gonorrhea is actually a puppeteer and directly physically triggering specific biological programs, a biological outcome, onto human cells,” he said.
As all superpowers have a weakness so do the pili, and Dr. Biais believes that N. gonorrhea’s power can be turned against it. When pili are stretched, they reveal new regions that are vulnerable to the immune system. Previous pili-based vaccines have not worked, possibly because they failed to take the force-mediated shift into account. Pre-stretched pili could be more effective. And because type-four pili are common to many different bacteria, one treatment could have many applications.
“Using this as a potential vaccine, you have the chance of killing more than one bird with one stone,” said Dr. Biais.
His thesis complete, Dr. Santos is already looking towards next-generation cell scaffolds that can replicate the body as a 3D structure rather than a flat surface, which he believes will be ideal for studying the body. “For hundreds of years biologists and doctors, people, have been looking at diseases from a biochemical point of view, and that is over,” he said. “If you want to understand how these diseases work in the lab, you really need to put the mechanical aspect into place.”
Dr. Dustin believes that the importance of rigidity is only the first step to truly understanding how cells interact with force. “We’ve basically found some interesting modulatory effects in our exploration over the past nine years,” he said. “But we don’t understand all of the things that we need to understand to realize that potential.”
Dr. Biais is unabashedly optimistic: “The sky’s the limit with what we can do with mechanobiology,” he said.
As these scientists toil away, poking, prodding and pulling in an attempt to unravel the mysteries of cellular mechanics, their work stands the chance of one day turning science fiction treatments into reality.
May the force, quite literally, be with them.

Leave a Reply
You must be logged in to post a comment.